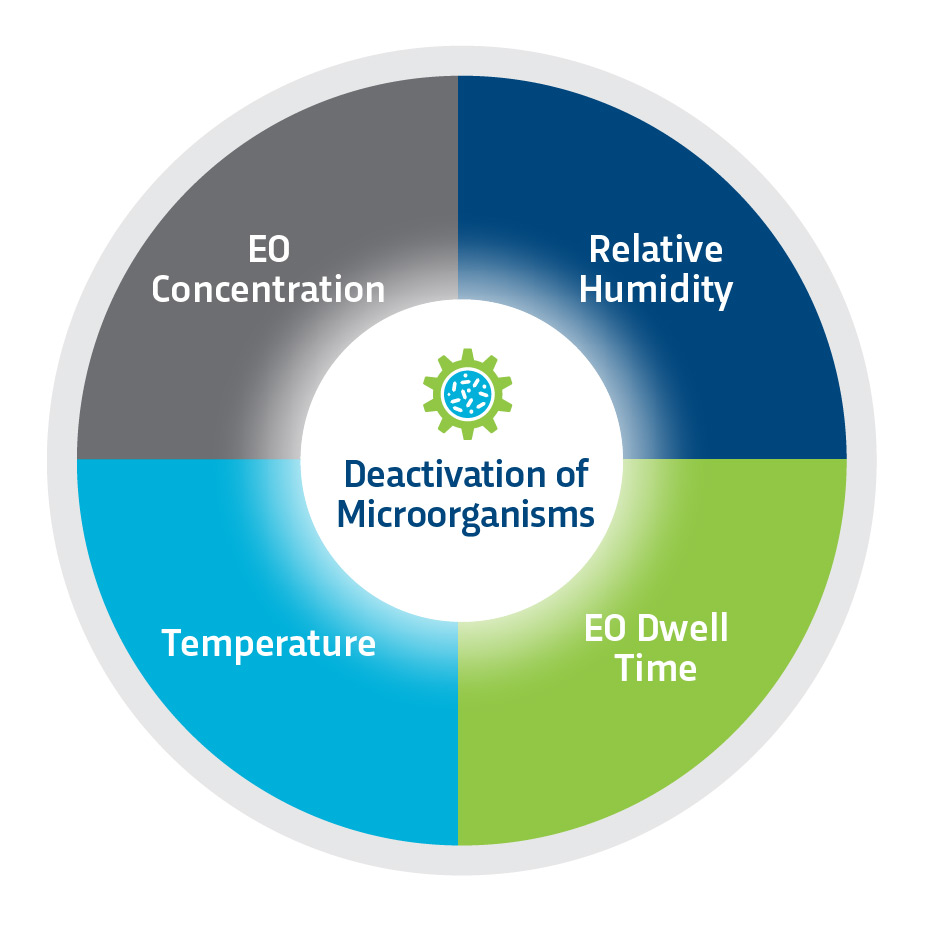
Critical Parameters for Effective Ethylene Oxide Sterilization
Based on a gas diffusion process, Ethylene Oxide (EO or ETO) is capable of sterilizing and rendering products free of viable microorganisms. Sterility occurs when an EO gas molecule reacts with and destroys the microbial DNA. The process requires the simultaneous control of four variables, but interdependent parameters: gas concentration, temperature, relative humidity, and time of exposure. ETO sterilization effectiveness depends on its ability to freely diffuse through a product and packaging. All products must be placed in breathable packaging that allows gas to penetrate the sterile barrier and reach all surfaces of the device or product.
Ethylene Oxide Sterilization is Best Suited For
ETO Sterilization is considered the sterilization method with the broadest application available for medical products and medical devices due to its effectiveness at lower temperatures and its general compatibility with a diversity of materials, resins, and product types, including:
-
- Polymer resin-based products
- Single-use medical devices
- Procedure kits
- Surgical trays
- Synthetic gowns
- Wound Care
- Medical Tubing
- Medical Tubing
- External terminal sterilization of sealed combination drug devices (filled syringes, drug-coated stents)












