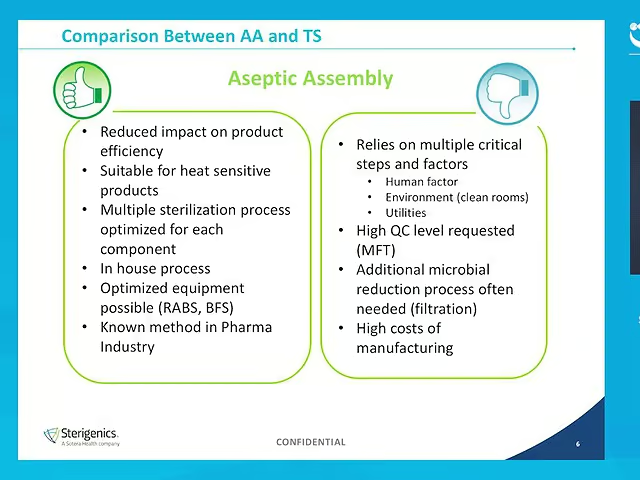
Regulations are clear and specific that, whenever possible, products intended to be sterile should be terminally sterilized in their final container. If terminal sterilization is not possible, only then should filtration or aseptic assembly be applied. This 2018 CPhI WW Pharma Insight Briefing compares aseptic assembly and terminal sterilization and suggests a structured approach for the assessment and selection of the optimal terminal sterilization method for your Pharma product.